
The information contained in this website is for European healthcare professionals only
I understand and confirm I am an EU healthcare professional
Read more >
Read more >
I am not an EU healthcare professional
Read more >
Read more >
▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
Overview of Totality of Evidence
MVASI® IS BACKED BY COMPREHENSIVE TOTALITY OF EVIDENCE1,2
WITH NO MEANINGFUL DIFFERENCE DEMONSTRATED VS. AVASTIN®, MVASI® HAS BEEN APPROVED BY THE EUROPEAN COMMISSION FOR USE IN BEVACIZUMAB-ELIGIBLE PATIENTS WITH THE SAME TUMOUR TYPES AS AVASTIN®*1-3
| Totality of Evidence In line with EMA scientific guidelines on biosimilars4 |
No meaningful difference... | ...compared to Avastin® |
|---|---|---|
ANALYTICAL CHARACTERISATION Structure-Function Analysis |
Same amino acid sequence, same strength, highly similar structure and function |
|
IN-VIVO STUDIES Non-clinical PK/PD and toxicology assessment |
Similar toxicology in cynomolgus monkeys Similar activity in epidermoid and colon cancer xenograft models |
|
| Similar PK/PD profile using US and EU regionally approved comparators | ||
CONFIRMATORY CLINICAL TRIAL Efficacy, safety, immunogenicity |
No clinically meaningful differences vs. Avastin® in a comparative phase 3 trial in NSCLC |
Note: Aim of a biosimilar is to demonstrate a high degree of similarity NOT independently re-establish safety and efficacy
†The MVASI® therapeutic indications do not include use in combination with paclitaxel for patients with platinum-resistant recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer.
Comparative Equivalency Trial (MAPLE)
MVASI®: COMPARATIVE EQUIVALENCY TRIAL (MAPLE) - STUDY DESIGN2,5

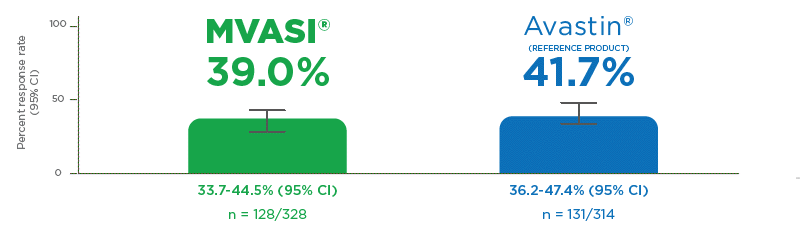
MVASI®: EQUIVALENT EFFICACY VERSUS AVASTIN®
OBJECTIVE RESPONSE RATE (ORR)2,5
PRIMARY ANALYSIS OF RISK RATIO (RR) AND SECONDARY ANALYSIS OF RISK DIFFERENCE (RD)2,5
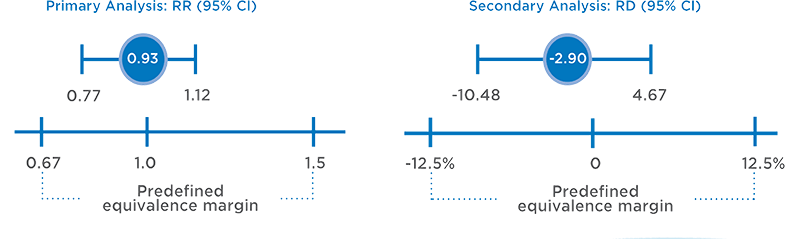
References:
- MVASI® (bevacizumab) Summary of Product characteristics. August 2020.
- EMA. Committee for Medicinal Products for Human Use (CHMP). MVASI Assessment Report, EMA/798844/2017.
- Avastin® (bevacizumab) Summary of Product characteristics. March 2020.
- EMA. Scientific guidelines on biosimilar medicinal products. Available at: https://www.ema.europa.eu/en/human-regulatory/research-development/scientific-guidelines/multidisciplinary/multidisciplinary-biosimilar [accessed 14 January 2020].
- Thatcher N, Goldschmidt JH, Thomas M, et al. Efficacy and Safety of the Biosimilar ABP 215 Compared with Bevacizumab in Patients with Advanced Nonsquamous Non-small Cell Lung Cancer (MAPLE): A Randomized, Double-blind, Phase III Study. Clin Cancer Res. 2019;25(7):2088-2095.
