
The information contained in this website is for European healthcare professionals only
I understand and confirm I am an EU healthcare professional
Read more >
Read more >
I am not an EU healthcare professional
Read more >
Read more >
▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
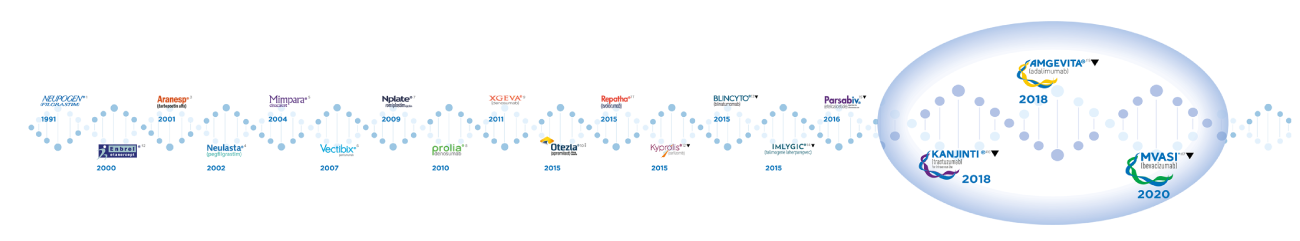
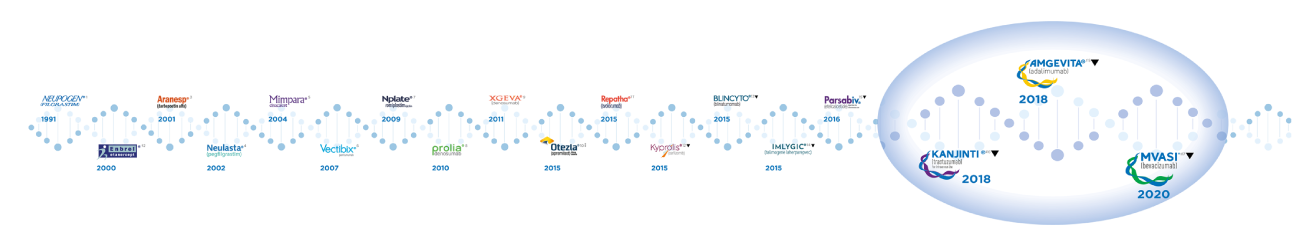
Amgen®’s Biosimilars
MVASI® IS A NEW CHAPTER IN AMGEN®’S LEGACY OF INNOVATOR AND BIOSIMILARS DEVELOPMENT*1-17

×


▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Healthcare professionals are asked to report any suspected adverse reactions.
MVASI® IS THE THIRD BIOSIMILAR THAT AMGEN® IS LAUNCHING IN EUROPE‡
2018‡
KANJINTI®▼
(trastuzumab) §16
(trastuzumab) §16
2018‡
AMGEVITA®▼
(adalimumab) §15
(adalimumab) §15
2020
MVASI®▼
(bevacizumab) §17
(bevacizumab) §17
*Dates indicate years of first market authorization by EMA/EC, except for biosimilar products‡.
†The marketing authorisation for Enbrel® is held by Pfizer in Europe.
‡Dates for biosimilar products indicate year of first commercial product availability in at least one European country. Note that for biosimilars market authorization dates and commercial launch dates can differ significantly.
§See SmPCs for full description of indications.
Heritage in Solid Tumours
MVASI®: CONTINUOUSLY STRENGTHENING OUR PRESENCE IN KEY ONCOLOGY AREAS3,4,6,9,16,17
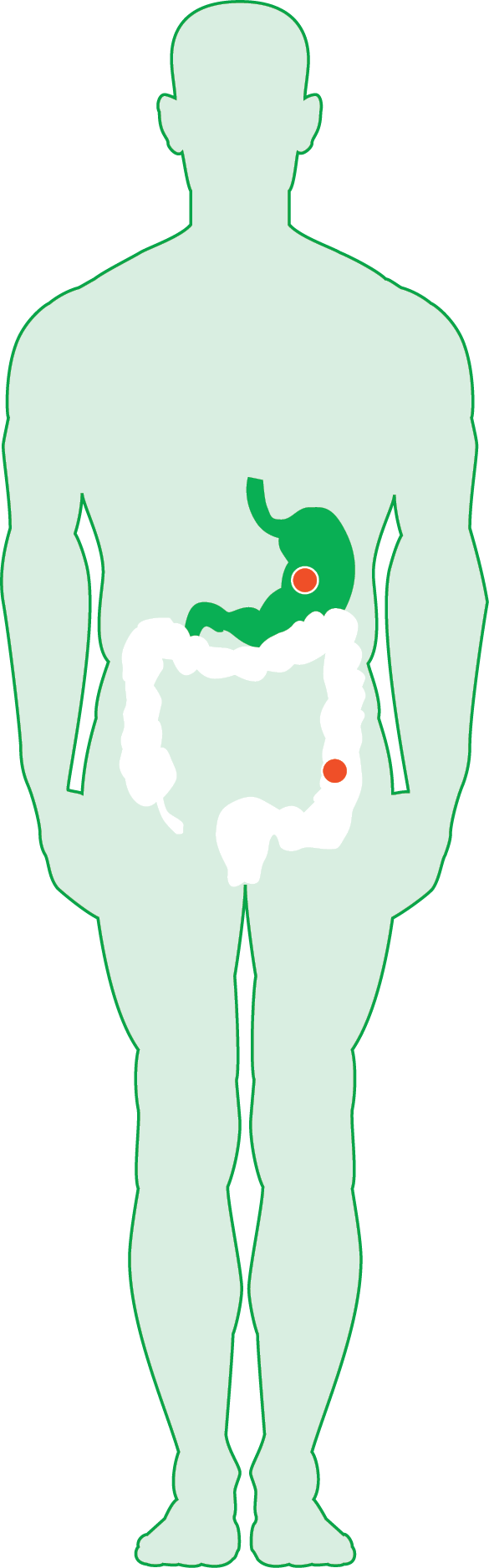
| Gastro Intestinal Cancer Typesǁ | ||||
|---|---|---|---|---|
 |
||||
| Since 2007* Vectibix®6 |
Since 2018* KANJINTI®16 |
Since 2020* MVASI®17 |
||
| mCRC | ||||
| Gastric Cancer | ||||
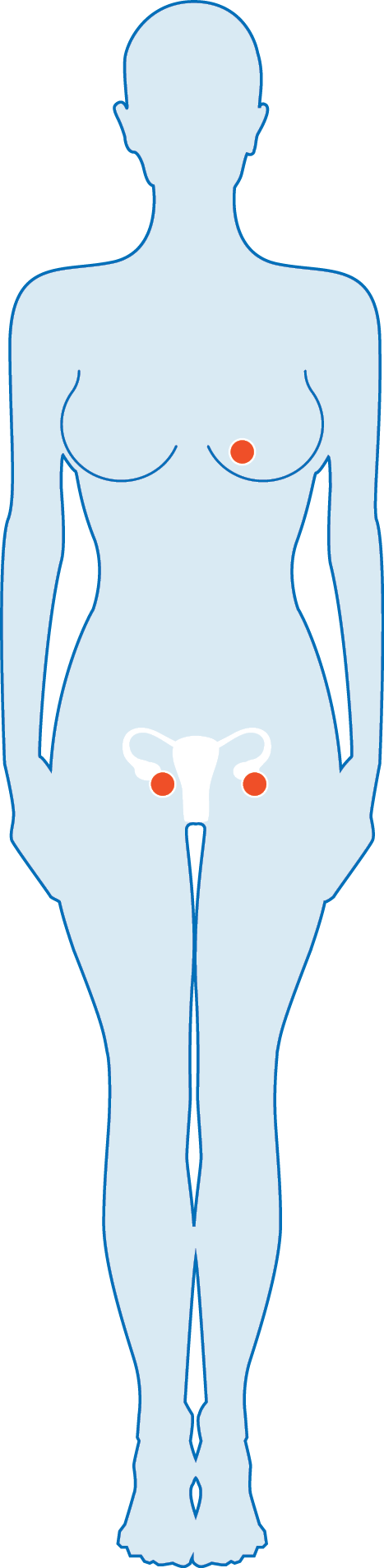
| Gynaecological Cancer Typesǁ | ||||
|---|---|---|---|---|
 |
||||
| Since 2011* XGEVA®9 |
Since 2018* KANJINTI®16 |
Since 2020* MVASI®17 |
||
| Breast Cancer | ||||
| Ovarian Cancer | ||||
| Bone Metastasis (advanced malignancies) |
||||
Supportive care: Aranesp® and Neulasta®3,4
*Dates indicate first year of commercial availability
Amgen®: TARGETED SOLUTIONS FOR ALL YOUR mCRC PATIENTS6,17
SOLUTIONS FOR ALL YOUR FIRST LINE mCRC PATIENTS, REGARDLESS OF RAS/BRAF STATUS AND TUMOUR SIDEDNESS6,17,19
ǁSee SmPC for full description of indication.
mCRC = metastatic colorectal cancer.
Manufacturing and Supply
ALL AMGEN® BIOSIMILARS ARE DEVELOPED AND PRODUCED USING THE SAME IN-HOUSE EXPERTISE, RIGOUR, AND INFRASTRUCTURE AS OUR INNOVATIVE BRANDS¶15,16,21,23
AMGEN® HAS FULL CONTROL OVER THE END-TO-END MANUFACTURING PROCESS21,23
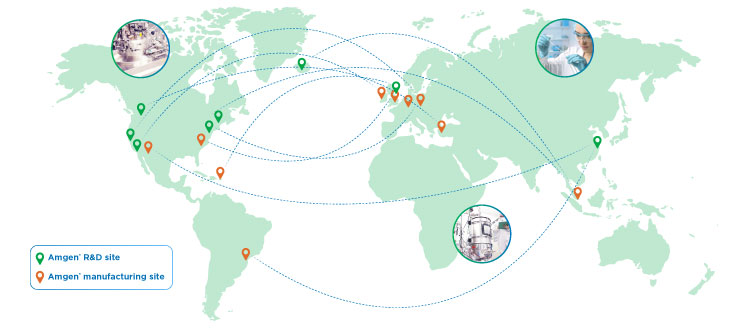
AMGEN® HAS A STRONG TRACK RECORD OF QUALITY MANUFACTURING AND RELIABLE DRUG SUPPLY22
AMGEN® IS COMMITTED TO ENSURING THAT ALL PRODUCTS ARE RELIABLY SUPPLIED TO EVERY PATIENT, EVERY TIME
> 99%
on time in full**22
deliveries from
2012-2017
on time in full**22
deliveries from
2012-2017
AMGEN®'S SUPPLY COMMITMENT |
|
|---|---|
 |
|
All stock and supply for Europe is labelled and packaged in Europe22 |
Our 100-country manufacturing and distribution network ensures patients get the medicines they need22 |
| Every unit adheres strictly to the same processes and quality-control measures |
|
We invest heavily in risk mitigation on all levels to ensure supply and safety |
|
¶The point-to-point connectors shown on the map are representational (not actual) in nature and are for the purpose of illustrating Amgen®’s fully-connected global network only.
**Displayed as average OTIF from 2012-2017. On time and in full (OTIF) is an industry standard to measure order delivery performance to our customers. The remaining < 1% consist of returns & damaged products not resulting in interrupted supply or any patient impact.22
References:
- NEUPOGEN® (filgrastim) Summary of Product Characteristics. May 2019.
- ENBREL® (etanercept) Summary of Product Characteristics. Nov 2019.
- ARANESP® (darbepoetin alfa) Summary of Product Characteristics. Jan 2020.
- NEULASTA® (pegfilgrastim) Summary of Product Characteristics. Nov 2019.
- MIMPARA® (cinacalcet) Summary of Product Characteristics. Aug 2019.
- VECTIBIX® (panitumumab) Summary of Product Characteristics. Jan 2020.
- NPLATE® (romiplostim) Summary of Product Characteristics. July 2019.
- PROLIA® (denosumab) Summary of Product Characteristics. July 2019.
- XGEVA® (denosumab) Summary of Product Characteristics. June 2019.
- REPATHA® (evolocumab) Summary of Product Characteristics. Nov 2019.
- KYPROLIS® (carfilzomib) Summary of Product Characteristics. Jan 2020.
- BLINCYTO® (blinatumomab) Summary of Product Characteristics. Nov 2019.
- IMLYGIC® (talimogene laherparepvec) Summary of Product Characteristics. Aug 2019.
- PARSABIV® (etelcalcetide) Summary of Product Characteristics. April 2019.
- AMGEVITA® (adalimumab) Summary of Product Characteristics. Oct 2019.
- KANJINTI® (trastuzumab) Summary of Product Characteristics. Nov 2019.
- MVASI® (bevacizumab) Summary of Product Characteristics. August 2020.
- Data on file.
- Arnold D, Lueza B, Douillard JY, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomized trials. Ann Oncol. 2017;28(8):1713-1729.
- Data on file.
- Amgen® Inc. Annual Report, Form 10-K 2018. Available at: https://www.sec.gov/Archives/edgar/data/318154/000031815419000008/amgn-12312018x10kq42018.htm [accessed 29 January 2020].
- Schipper R, Middelkoop K, Biniass B, Hippenmeyer J. Assessment of drug supply reliability in Europe by evaluating on time in full (OTIF) delivery. Poster presented at BOPA 21st Annual Symposium 12–14 October 2018, Birmingham, UK.
- Amgen® Biosimilars website. Available at: https://www.amgenbiosimilars.com/. Accessed 19 February 2020.
